Abstract
Patients with type 3 von Willebrand Disease (VWD) usually have markedly reduced FVIII/VWF levels and very severe bleeding manifestations but, because of their rarity, their bleeding phenotype is poorly described. We aimed at evaluating the distribution of bleeding symptoms in patients with type 3 VWD, comparing them with previously available data from a cohort of type 1 patients, and describing site-specific clustering of bleeding symptoms in these patients. We analyzed clinical data from the type 3 Von Willebrand International RegistrieS Inhibitor Prospective Study (3WINTERS-IPS),a no-profit, investigators initiated, multicenter, European-Iranian observational, retrospective and prospective study on patients with diagnosis of type 3 VWD. Aims of the 3WINTERS-IPS is 3-fold: a) to identify the main phenotypic and molecular characteristics of a large cohort of VWD patients; b) to evaluate the risk factors responsible for the severe bleeding phenotype; c) to assess the efficacy and safety of the treatment with VWF concentrates with or without FVIII including the risk of anti-VWF antibodies. Retrospective information on bleeding symptoms at presentation was collected using the MCMDM-1 VWD bleeding questionnaire, and bleeding severity summarized as bleeding score. Individual bleeding symptoms were considered as relevant when having a score >1 (hence requiring medical attention). Data was compared with that retrieved from the MCMDM-1 VWD study database on patients affected by type 1 VWD (index cases and affected family members).
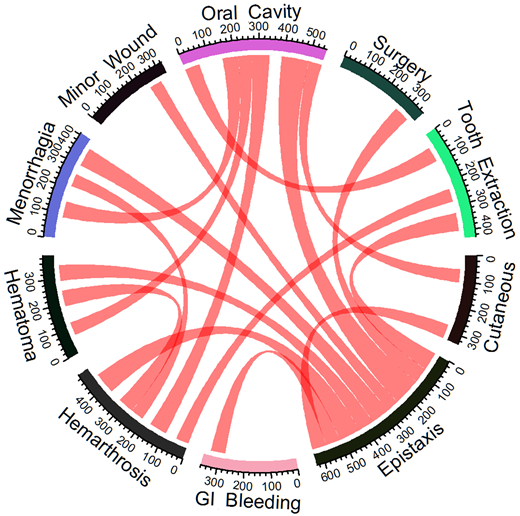
The study enrolled a total of 260 patients, of which we analysed 243 patients with available bleeding score at recruitment. The median age at study inclusion was 29 years (interquantile range, 26.5 years); 140 were females (53.8%). There were 108 patients of Iranian descent, while the remaining of patients were from Europe. The median number of bleeding symptoms was 5, and the median bleeding score was 15 (interquantile range, 13). Only 7/243 patients (2.8%) had a single bleeding symptom. Epistaxis was the most frequent relevant symptom, being present in 195 patients (80.2%), followed by menorrhagia in 99 females (70.7%). Males had a higher frequency of hemarthroses and hematomas than females (53.4% vs 42.1% and 40.8% vs 27.1%, respectively). When comparing the clinical presentation of type 3 vs. type 1 VWD, clearly increased bleeding scores were evident for all age-classes and even in paediatric cases. The association between symptoms having a relative frequency >20% is presented in the circle diagram, showing that some symptoms appeared to cluster with others in a variable degree (e.g., menorrhagia with epistaxis, hemarthrosis or oral cavity bleeding; post-extraction bleeding again with epistaxis, hemarthrosis or oral cavity bleeding; surgical bleeding or gastrointestinal bleeding with epistaxis alone).
These findings confirm the severity of type 3 VWD and extend the knowledge of symptoms distribution in the widest available cohort of type 3 VWD patients.
Tosetto:Stago, Novo-Nordisk, BMS: Speakers Bureau; Werfen: Other: Member of Advisory Board, Speakers Bureau. Berntorp:Octapharma: Consultancy; CSL Behring: Consultancy; Shire: Consultancy, Other: honoraria for lecturing . Eikenboom:CSL: Research Funding. Mazzucconi:Baxalta-Shire: Consultancy, Speakers Bureau; Bayer: Consultancy, Speakers Bureau; Novartis,: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Novo Nordisk: Consultancy, Speakers Bureau; CSL Behring: Consultancy, Speakers Bureau. Oldenburg:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Biogen Idec: Honoraria, Membership on an entity's Board of Directors or advisory committees; Chugai: Honoraria, Membership on an entity's Board of Directors or advisory committees; Grifols: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Shire: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Octapharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo Nordisk: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Behring: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Biotest: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Swedish Orphan Biovitrum: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Peyvandi:Kedrion: Consultancy; Ablynx: Other: Member of Advisory Board, Speakers Bureau; Shire: Speakers Bureau; Roche: Speakers Bureau; Shire: Speakers Bureau; Kedrion: Consultancy; Kedrion: Consultancy; Ablynx: Other: Member of Advisory Board, Speakers Bureau; Grifols: Speakers Bureau; Roche: Speakers Bureau; Octapharma US: Honoraria; Octapharma US: Honoraria; Sobi: Speakers Bureau; Ablynx: Other: Member of Advisory Board, Speakers Bureau; Kedrion: Consultancy; Shire: Speakers Bureau; Roche: Speakers Bureau; Roche: Speakers Bureau; Novo Nordisk: Speakers Bureau; Shire: Speakers Bureau; Ablynx: Other: Member of Advisory Board, Speakers Bureau; Grifols: Speakers Bureau; Shire: Speakers Bureau; Ablynx: Other: Member of Advisory Board, Speakers Bureau; Grifols: Speakers Bureau; Novo Nordisk: Speakers Bureau; Octapharma US: Honoraria; Octapharma US: Honoraria; Sobi: Speakers Bureau; Grifols: Speakers Bureau; Grifols: Speakers Bureau; Kedrion: Consultancy; Sobi: Speakers Bureau; Roche: Speakers Bureau; Novo Nordisk: Speakers Bureau; Novo Nordisk: Speakers Bureau; Novo Nordisk: Speakers Bureau; Sobi: Speakers Bureau; Octapharma US: Honoraria; Sobi: Speakers Bureau. Schneppenheim:SHIRE: Consultancy; CSL Behring: Consultancy. Tiede:Alnylam, Bayer, Biogen Idec, Biotest, Bristol-Myers-Squibb, Boehringer Ingelheim, CSL Behring, Leo Pharma, Novo Nordisk, Octapharma, Pfizer, Roche, Shire, and SOBI: Consultancy; Alnylam, Bayer, Biogen Idec, Biotest, Bristol-Myers-Squibb, Boehringer Ingelheim, CSL Behring, Leo Pharma, Novo Nordisk, Octapharma, Pfizer, Roche, Shire, and SOBI: Honoraria; Alnylam, Bayer, Biogen Idec, Biotest, Bristol-Myers-Squibb, Boehringer Ingelheim, CSL Behring, Leo Pharma, Novo Nordisk, Octapharma, Pfizer, Roche, Shire, and SOBI: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.